Abstract
Thrombosis is a major cause of morbidity and mortality in polycythemia vera (PV) and essential thrombocythemia (ET). The mechanistic basis of thrombosis in PV/ET, however, is unknown. To better understand the pathophysiology of thrombosis in PV and ET, we first studied transcript levels of selected thrombotic, inflammatory and hypoxia- inducible factor (HIF) pathway genes in granulocytes and platelets of PV and ET patients with and without thrombosis. Genes selected for the study included: tissue factor (F3); P-selectin (SELP); serpin peptidase inhibitor clade E member 1 (SERPINE1, encoding plasminogen activator inhibitor I, PAI1); thrombospondin 1 (THBS1); interleukin 1 receptor associated kinase 1 (IRAK1); interleukin 1 receptor accessory protein (IL1RAP) and HIF-regulated genes: vascular endothelial growth factor A (VEGFA) and solute carrier family 2 (SLC2A1, encoding glucose transporter 1). We have previously reported at this meeting that PV and ET patients with a history of thrombosis had higher transcripts of F3, SERPINE1, IL1RAP, VEGFA and SLC2A1 compared to those without thrombosis. We also performed unbiased total RNA sequencing of platelets and granulocytes (Gangaraju R et al Blood, 2016;128:3143).
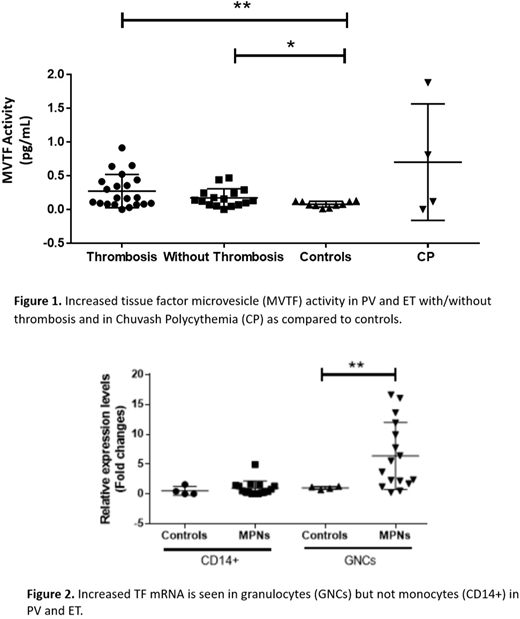
Tissue factor (TF) is the principal initiator of coagulation in vivo. The presence of TF transcript in leukocytes and platelets may or may not reflect the translated protein level. Furthermore, TF functional activity is modulated by encryption. Therefore, we proceeded to evaluate the functional activity of microvesicle-associated TF (MVTF) in the plasma of 10 ET and 33 PV patients considered to have high thrombotic risk (Tefferi A, Barbui T Am J Hematol. 2017;92(1):94-108). TF activity was measured in MVs collected by centrifugation of patient plasma to 20,200 xg by a two-step FXa generation assay with and without an inhibitory TF antibody to determine the contribution of TF to FXa generation (Owens 3d et al. Circ Res. 2011;108(10):1284-97). We found significantly increased levels of MVTF activity in PV and ET compared to normal controls (Figure 1). However, MVTF levels in PV and ET patients with and without thrombosis were comparable (Figure 1).
In the vasculature, leukocytes can synthesize TF (upon stimulation) and it has been shown that monocytes, not neutrophils, are the principal source of TF under normal conditions (Osterud B, Thromb Res. 2010;25 Suppl 1:S31-4). MPN granulocytes, in contrast to normal granulocytes, had increased levels of TF transcripts, a novel and important finding of as yet undetermined significance (Figure 2).
Since TF synthesis is regulated by hypoxia-inducing factor-1 (HIF-1) (Rolfs et al. J Biol Chem. 1997;272(32):20055-62), we also examined MVTF activity in the plasma of Chuvash polycythemia (CP) patients. These patients have a germline VHLC598T mutation in the negative regulator of HIFs, the von Hippel Lindau gene (VHLC598T), and as a result they have increased levels of HIF-1, HIF-2 and transcripts of a vast array of HIF-regulated genes. CP subjects have an even higher propensity for arterial and venous thrombosis than PV. As predicted, some CP plasmas also demonstrated elevated levels of MVTF activity (Figure 1). In conclusion, hypoxia-induced increased levels of plasma MVTF activity may play a role in the increased thrombotic risk of PV and ET. TF joins TSP-1 (Sergeueva et al. Haematologica. 2017;102(5):e166-e169) and protein S (Pilly et al. Blood 2018;132(4):452-455) as a potential HIF-regulated mechanism of thrombotic risk in patients with PV/ET and CP. Granulocytes may also be a source of hypoxia-induced TF in these patients. The hypoxia-mediated upregulation of thrombotic risk is further underscored by the observation that PV patients living in moderate hypoxia at Salt Lake City have a higher risk of thrombosis than those living at sea level (Baltimore MD) in multivariate analysis (Zangari et al, Blood Coagul Fibrinolysis 2013; 24(3):311-316). The data described here may facilitate identification of novel targets and their therapies including the use of HIF-1 inhibitors such as digoxin to prevent thrombosis in these patients (Zhang et al. PNAS 2008;105(50):19579-86).
Key:UniQure BV: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal